Update: To read other articles in this series, click here.
Over the last few decades, scientists have learned a lot about how life interacts with the air, land, and sea. And in the process, they’ve made observations that have demonstrated beyond any reasonable doubt that the increasing carbon dioxide in the air is from people burning coal, petroleum, and natural gas.
So how did the scientists put together all the pieces to make a complete conclusion? They started with an understanding of how plants use carbon during photosynthesis. That knowledge showed that the increased carbon dioxide in the air was from plants. Then they formulated some guesses as to where that much plant-based carbon dioxide could come from and, by process of elimination and careful accounting, determined that the source was human consumption of fossil fuels.
But it all starts with the concept of isotopes.
What is an isotope?
If you’ve heard of uranium 235, depleted uranium, light or heavy water, deuterium, or carbon dating, you’ve already been exposed to the concept of an “isotope.”
Every element on the periodic table is made up of a number protons, electrons, and neutrons. The number of protons determine what the element is – hydrogen (1) has one proton, helium has 2, lithium has 3, and so on. The number of electrons determine the electric charge of the atom, and whether it will conduct electricity or react chemically with another atom. And the number of neutrons an given atom has determines the isotope of that element.
Naturally-occurring uranium has two main isotopes, uranium-235 and uranium-238, also notated as 238U. 235U is less stable than 238U, and so it’s used as the fuel for nuclear power plants and the explosive material in fission bombs. The more stable 238U that’s left over from the uranium enrichment process is also known as “depleted uranium”. Light water has two hydrogen atoms bonded to one oxygen atom, while heavy water has deuterium atoms (hydrogen with an extra neutron) instead of standard hydrogen.
Carbon has three isotopes, carbon-12, -13, and -14. Carbon dating uses the rate at which carbon-14 decays to estimate how long ago a plant or animal died. Because all life we know of is based on carbon, the three main isotopes of carbon let scientists understand a lot about how life interacts with the physical world.
Carbon isotopes let us track how life changes the air, land, and sea
Plants use carbon and sunlight to make carbohydrates, the chemical fuel that enables plants, and the animals that eat plants, to live. And when plants make carbohydrates, they use any of the carbon isotopes that happen to be available. But carbon-13 and -14, being slightly heavier than carbon-12, need just a little more energy to turn into a carbohydrate. Since plants are all about getting the most energy they can out of the sun, they convert more carbon-12 into carbohydrates than they do carbon-13, or carbon-14.
Scientists have measured the physical ratio of carbon-13 to carbon-12, often written as 13C/12C, and it’s approximately 1 atom of carbon-13 to every 99 atoms of carbon-12. Because plants use slightly more carbon-12 than carbon-13 as they make carbohydrates, scientists can track how plant life affects changes the ratio 13C/12C. In the course of photosynthesis, plants deplete the amount of carbon-12 in the atmosphere, increasing the 13C/12C ratio.
But when living things die and decay or are burned, all that extra carbon-12 is emitted back into the atmosphere, lowering the 13C/12C ratio by increasing the amount of carbon-12.
Scientists have measured a noticeable drop in 13C/12C, as shown in the top figure, since the early 1990s. Scientists have measured drops in the ratio since at least the early 1800s by analyzing the amount of carbon-12 and carbon-13 in corals*. This drop in the 13C/12C ratio can only be a result of one thing – living things decaying or being burned. However, while we know what the source is in general, the change in the ratio itself isn’t enough to say for certain what the specific source is.
There are three possibilities for how the extra carbon-12 could be entering the atmosphere. It can be entering the atmosphere from the ocean. It could be coming from the decay of lots of recently living plants and animals. Or it could be coming from the burning of plants and/or fossil fuels.
The extra carbon isn’t coming from the ocean
The ocean stores a lot of carbon and most of it came from life at one point or another. Algae convert that carbon dioxide into carbohydrates that, just like any plant matter, has lower 13C/12C ratio. When the algae die, they slowly settle to the bottom of the ocean, where they decay. As they decay, some of that carbon turns into carbon dioxide and gets dissolved in the water. Once in the water, that carbon dioxide can circulate via ocean currents and rise up to the ocean’s surface where it can go back into the atmosphere. But scientists know that this isn’t where the added carbon dioxide in the air is coming from.
The way we know is that scientists can measure the amount of carbon dioxide dissolved in the water. And when they measure it, they find that the amount of carbon dioxide in the water is not only increasing, it’s increasing nearly everywhere.
If the added carbon dioxide in the air was from the deep ocean, we would expect that the amount of carbon dioxide would be increasing at those places where deep ocean water rises to the surface, while the amount of carbon dioxide in the water would be decreasing everywhere else. Since the amount of carbon dioxide is increasing everywhere by about the same amount, that means the carbon dioxide has to be coming from the air and going into the water, not the other way around.
The extra carbon isn’t coming from land plants either
So long as plants are alive and growing, they absorb carbon dioxide from the atmosphere and store it away. But when a plant dies, it slowly releases that stored carbon back into the air as rots away The process isn’t fast, but it is largely in balance. This means that the carbon emitted by a rotting tree will be almost identical to the carbon absorbed by the tree while it was alive. After all, it’s not like a tree that stores 5 tons of carbon can emit more carbon that it has stored in its limbs, leaves, and roots.
It is, however, possible that not all the carbon will be released, resulting in some amount of storage of carbon in the land. Decaying leaves, plant roots, and tree limbs are incorporated into the soil or turned into peat or permafrost, where the carbon remains stored until something triggers a release. And just as the deep ocean stores a significant amount of carbon, so too do soils.
So it’s hypothetically possible for all the increase in life-sourced carbon dioxide to be coming from soils. But scientists have tested this hypothesis specifically, setting up parcels of land and very carefully tracking how much carbon dioxide was absorbed or emitted over the course of several years. Scientists then used those observations to estimate how much carbon dioxide is being added to, or subtracted from, the air by plants.
They found that plants are absorbing more carbon dioxide than they’re emitting, completely contradicting the hypothesis that increases in atmospheric carbon dioxide are due to land emissions.

Figure 3 – Increasing CO2, decreasing 13C/12C, decreasing
O2, and increasing fossil fuel consumption
The carbon is coming from the burning of fossil fuels
Since the extra carbon dioxide can’t be from the ocean, and it can’t be from soils, it must be from burning living plants or fossil fuels. While the simple process of elimination leaves this hypothesis as the last one standing, there are two ways to test this hypothesis as well, and both ways confirm it.
The first way to test the hypothesis to measure the amount of oxygen in the air and see if it’s decreasing. If it is, then the decrease is likely due to combustion.
If you’ve ever done the candle in a jar experiment, then you know how combustion works. The experiment goes like this:
- Find a candle and a jar or clear bowl that will fit over the candle.
- Light the candle and then place the jar or bowl over it, and wait.
- After a little while, the candle will snuff itself out.
The reason the candle goes out is because the flame eventually uses up all the oxygen, and without oxygen combustion isn’t possible. If you measured the air in the jar before and after the candle had gone out, you’d find that it had almost no oxygen after the candle went out and there was a great deal of extra carbon dioxide.
Since scientists have been able to take accurate measurements of the amount of oxygen in the air since the early 1990s, the amount of oxygen in the air has fallen slightly, as shown in Figure 3. This decrease shows that the burning of plants and/or fossil fuels is responsible for the dropping carbon-13/carbon-12 ratio.
The second way to test the combustion hypothesis is to count all the fossil fuels combusted in a year, convert those barrels of petroleum, tons of coal, and cubic feet of natural gas into carbon emitted into the air, and then compare that to the increase in carbon dioxide.
Scientists have done just this, and they’ve learned that fossil fuels add more carbon dioxide to the air every year than the increase that is measured. This means that the extra carbon dioxide has to be going somewhere, and there are only two places where it could be going – into the ocean and into plants. And if you recall, scientists have measured that the amount of carbon dioxide in the ocean is increasing globally, and that land plants are absorbing carbon too.
Putting it all together
So what do we know now? We know that the added carbon dioxide in the air is from a source that is, or was, alive. We know that human burning of fossil fuels adds a lot more carbon dioxide to the atmosphere than what stays there. We know that the only two places that extra carbon dioxide could be going is into the ocean and into plants. We know that the oceans are absorbing carbon dioxide, and we know that that land plants are too.
Combined, these observations prove beyond any reasonable doubt that the increase in atmospheric carbon dioxide is due to fossil fuel combustion.
* Corals are used because they live so close to the ocean surface that the amount and isotopes of carbon in the water closely match what’s in the air.
Image Credits:
Figure 1: NASA
Figure 2: NOAA ESRL
Figure 3: IPCC AR4 Chapter 2, figure 2-3
Categories: Education, Environment/Nature, Science/Technology





Yep,
we know fur-sure that
“This drop in the 13C/12C ratio can only be a result of one thing ..”
we know! we know!
On the other hand,
a) what is solar cycle 25 doing to do?
don’t know? how about solar cycle 24 max?
NASA says 2010 and really big, no it’s 2011, no it’s 2012, no it’s 2013…
b) what, exactly, is the ocean uptake/out gassing of co2 and how does it relate to the totally unpredictable solar cycle?
Oh, that’s “can’t possibly affect Mauna Loa” (the only really stable co2 measurement),
just cuz other ones wander all over the map…..
Haaa, Haaa, Haaaa!
cuz we know fur-sure!
The solar cycle has no measurable influence influence in the amount of carbon dioxide in the atmosphere at this time. Therefore, discussions of the solar cycle are off topic for this post, which is about the origin of CO2, not solar cycles (or climate sensitivity, or radiative forcing of CO2, or anything else)
Scientists have measured the amount of carbon dioxide flux at the air/ocean boundary, and the direction of flux is from the air into the water. As such, the ocean is not outgassing carbon dioxide. This is verifiable scientific fact.
Mauna Loa is not the only CO2 measurement, either for 13C/12C or for total CO2. I encourage you to familiarize yourself with the measurements at the following sites, all of which show CO2 increasing since reliable direct measurements began: http://cdiac.ornl.gov/trends/co2/sio-keel.html
“The solar cycle…”
(about which we are clueless)
“has no measurable….” (that we know of)
“influence on CO2…..”
=> you might want to look at the geographic spread of the CO2 “standards” sites.
seems to be a bit coupled to the Pacific Ocean.
“Scientists have measured…this is verifiable scientific face.”
Except, of course, that La Nina brings more cold, CO2 water to the surface in the tropics,
where, under the hot topic sun, some of the CO2, unmeasured, is released.
….and that is a verifiable, scientific fact.
Not to mention that cold water holds a bunch more CO2 than warm and there is some evidence that northern climes are the CO2 sink and southern climes are a source.
Very complex and not understood at all.
Naaah, can’t be a source of CO2!
A lot we don’t know, but we are sure!
Six of those sites are Pacific ocean coupled, yes. Three are not. However, it shouldn’t matter as carbon dioxide is a well-mixed gas in the atmosphere. However, if you prefer, look at the ESRL global mean carbon dioxide increase here The explanation of how this global mean is calculated is available here, and here’s the sites where atmospheric samples are taken in order to generate that mean (complete list available here).
So it’s not a corruption of the data and it’s not a spatial sampling issue. While La Nina might have an effect, it’s not having a major impact because that would be visible in the data. Similarly, there is no 11 or 22 year variability visible in the carbon dioxide data either, which would be necessary for solar cycles to be driving carbon dioxide levels.
And again, scientists have directly measured that the ocean is absorbing more carbon dioxide than it is releasing, measurable as a slightly reduction the the pH of the ocean, as shown below:
Again, it is verifiable scientific fact that the oceans are not the source of the increase in atmospheric carbon dioxide.
Yes,
no argument that the relatively constant TSI and IR would not show in the CO2 levels.
That said, the actual CO2 latitudinal distribution has an amazing “bump” around the equator.
From http://www.esrl.noaa.gov/gmd/ccgg/about/global_means.html, we have the 1980-09-16 and 2009-09-16 distributions (hmmm, wonder if you allow markup…)
That much “extra” coming from huge areas of ocean is a “big deal” and you would be hard pressed to say it was from “industry”.
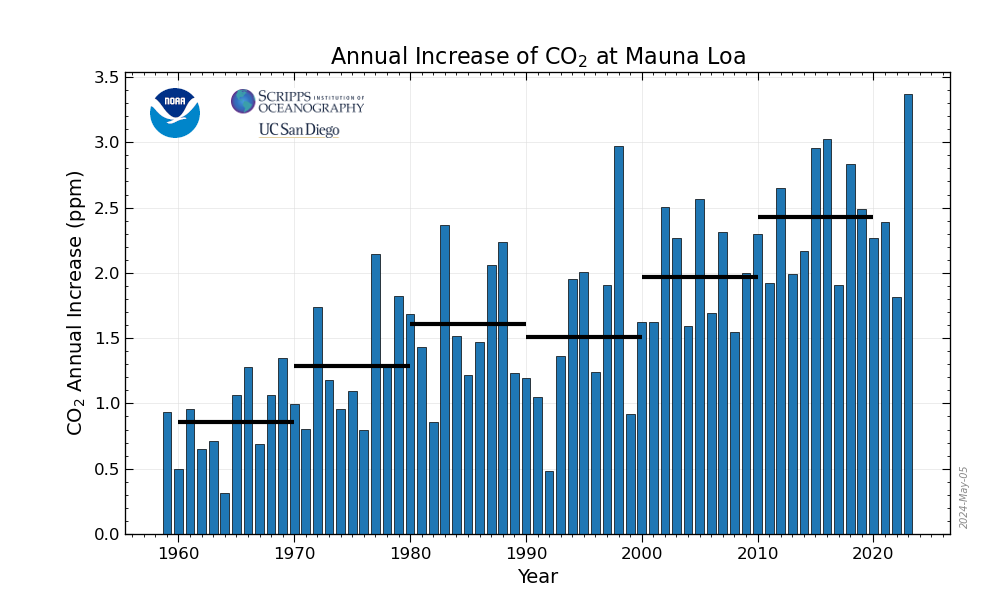
Then there is the “annual mean growth rate of CO2 at Mauna Loa
http://www.esrl.noaa.gov/gmd/ccgg/trends/mlo.html#mlo
that clearly shows the cycles of La Nina and El Nino where the warming water (which had been cold) releases more CO2, again by a huge area of ocean.
This doesn’t include that the CO2 cycle curves are just a bit early for the rationalization of “human heating in N. Hemisphere” and “plant growth in N. Hemisphere” but matches much better with TSI on Ocean.
But then, again, it only takes 300 years for the ocean to recover from a solar ice age and who there aren’t many research grants to study such unimportant things….
unless, of course, the Little Ice Age never happened, the CET is bogus, and Einstein’s generation could invent the atomic bomb but couldn’t read thermometers!
Here’s the “1980-09-16 and 2009-09-16” image you were talking about (sometimes markup works, sometimes it doesn’t. I know it works for authors, but I’m less sure about commenters, subscribed or not):
You are correct that there is a bump around the equator, and you are almost certainly correct that it’s due to upwellings either as a result of La Nina or from ocean currents. However, you’ll also notice that the bump is about the same size (1.5-2 ppm) from the north pole to the equator, and that the difference between the two poles is about the same as well (5 ppm in 1980 vs. 6 ppm in 2009. The entire curve has shifted up, and essentially equally. it still comes back to the point that scientists are observing the ocean absorbing carbon dioxide, not releasing it.
As for La Nina/El Nino, big dips and spikes in the annual mean growth rate of carbon dioxide appear to be associated with La Nina and El Nino respectively, but not always. For example, looking at the ENSO data here, you see that the 1998 was a big spike (because the strong El Nino hit starting in mid 1997) followed by a deep dip due to a La Nina that went from mid 1998 through early 2001. But if you look at 1991-1993, which were around El Nino years, there is actually a dip in the growth rate.
Furthermore, the annual mean growth rate is always positive, even in those years when less carbon dioxide is being added to the atmosphere, such as during the 1991-1993 period. Not only that, we know from history what DID cause the 1991-1993 dip – the collapse of the USSR and the associated energy production. What this suggests is that the annual mean growth rate of carbon dioxide may go up slightly due to El Ninos and down slightly due to La Ninas, but that the overall growth rate is not controlled by the ocean, your Central England Temperature and Little Ice Age comments notwithstanding.
But let’s turn this around. If the increase in carbon dioxide is from the ocean, then where is all of the fossil fuel-produced carbon dioxide magically disappearing to? Simple addition and subtraction proves that all the coal, oil, and gas that are burned every year is roughly double the amount observed remaining in the atmosphere. If all the increase is from the ocean, then that means all the fossil-fuel carbon dioxide is going somewhere that scientists haven’t discovered yet. Where is it going, and what’s your evidence?
Put up or shut up time – admit you’re wrong about the ocean being the source of the additional carbon dioxide, or provide incontrovertible evidence that supports your hypothesis. It better explain all the points I made in the OP above, however – decreasing 13C/12C ratio, increasing CO2 levels in the ocean, observed absorption of CO2 by land plants, and conservation of mass related to fossil fuel CO2. Because if it can’t match each and everyone one of those observed, known facts and calculations, then it’s wrong.
Also , make sure you explain the reduction in atmospheric oxygen, which wouldnt be happening if the carbon dioxide increase was due to oceanic outgassing.
Yep, the Pinatubo eruption in June 1991, cooling everything, couldn’t possibly have caused the oceans to release less CO2,
Must have been the USSR shutting down, big time, and not heating their homes in Siberia….
nope, never, couldn’t possibly, having rejected the theory that Little Ice Recovery outgasing is TSI dependent…. yep, can’t be, my dogma says so.
Yep, and there are tons of papers on the precise measurements of ocean uptake and release!
…tons of papers, tons…. there’re around here somewhere…..
so what if the area from 30 S to 30 N is mostly ocean, we know everything that is happening, in precise detail, to the 335,250,000 sq km of ocean!
So,
just cuz we don’t seem to have a clue, a theory, or anything, it must be true!
Remember, it takes only one little new piece of information, like CERN’s support of Svensmark’s theory, to totally falsify a theory that can’t predict it.
So, “put up or shut up” time, as you said,
=> unless you can refute Svensmark’s theory, and scope out all the “unknowns”,
then you have to admit there is “a problem”
(and “hand waving – it doesn’t matter” isn’t useful on the leading edge of reseach)
I’ll grant the possibility that Pinatubo had an impact, but you haven’t demonstrated it.
If the extra CO2 was from the ocean, then the ocean wouldn’t be absorbing carbon dioxide and it’s pH wouldn’t be dropping. If the extra CO2 was from the ocean due to thermal outgassing, the amount of oxygen in the air would be going up or remaining constant, not dropping. And you still haven’t addressed where all the fossil fuel carbon dioxide would be getting stored, as required by simple conservation of mass, if it wasn’t the cause for the increasing atmospheric and oceanic carbon dioxide.
I asked you to present a hypothesis that was in agreement with all the facts, and you failed to do so. Instead, you shifted the goalposts to a new argument without admitting you can’t defend your prior argument. This demonstrates that you are not arguing in good faith, and that your opinions are more important to you than scientific facts. This makes you a denier of climate science.
If you wish to have a productive, open, good faith discussion, by all means post again. However, if you fail to meet these standards, your posts will be published.
Well, Brian,
I ran this by my friend, (she’s the smartest person I’ve encountered even if she isn’t on salary to me) and she just laughed.
“‘prove me wrong’ is not at all science, it is religion. It takes only one obnoxious fact to falsify a theory”
She then noted, in this context, that AGW theory must (and hasn’t) explained why, in its domain, that “the entire civilized world shut down/used almost no CO2 generating fuels in 1964, 1991, 1999, and much less than normal in 1982 (and other years) according to the Mauna Loa measurements of delta by year… and SST didn’t change that much, so it ain’t the ocean.”.
=> explosive volcanic eruption can explain the drop in temperature record but not the drop in increment of the CO2 record.
She also opined that the “ocean is really huge and the cute little ‘toy’ picture in the article, representing somewhat about 1% of the earth’s surface (guess, not calculation given that 70-90 NS, and 5-15NS are not at all like the picture), is a bit trite.”
So, my theory is that we don’t really have a clue.
It is very clear from the variance in the CO2 records, that there is a huge discrepancy between the simplistic “people add all CO2”.
The bully boy antics that have been displayed in defense of AGW theory, which are classic male domination techniques, give doubt as to the veracity of the proponents of that theory and concern about selective reporting in the research papers.
If you think we actually have a clue, then showing where in the AGW theory the drastic drop in CO2 additions can be accounted for would be the trick.
I ran this by my incredibly smart friend (IQ over 200) and he thinks your friend is actually your mother.
Aaaaand, more bad faith debate techniques from Lol, this time the “appeal to misleading authority” fallacy. Any anonymous authority is inherently misleading.
You think “we have no clue.” The science says otherwise – it creates a logically and self-consistent explanation for all of the data available to date. Natural variations cause fluctuations, of course, but don’t change the overall trend or the ultimate source of the trend.
“So, my theory is that we don’t really have a clue.”
I see this very often from deniers of climate science. They say “we” when they mean “I”.
“LOL in Oregon” seems to be mainly concerned with coming across as extravagantly obnoxious, so much so that it’s not really that clear what he/she is actually arguing. That the rise in CO2 is from ocean outgassing? I think that’s the claim. But in addition to all the evidence already presented that shows this is not the case, you could also consider that going by glacial records of ΔCO2 vs. ΔT, you would need a global temperature rise of about 20K to get the oceans warm enough to release the 110ppm that CO2 concentrations have risen by.
Do you think that much global warming has happened, “LOL in Oregon”?
Doesn’t some Carbon 12 come out of the ground, either in the methane or directly as CO2 from soil organisms? If so, then the heavier isotopes of Carbon, would be retarded by the long path to the surface. This would throw off the isotope ratios and could be happening due to the increase in atmospheric CO2.
CptWayne, carbon does come out of the ground, both as methane and as carbon dioxide. Methane does get converted into carbon dioxide in the atmosphere, but it isn’t a major source of overall carbon dioxide since the increase are measured in parts per billion, while the direct increases in carbon dioxide are 1000x larger.
Direct emissions of carbon dioxide from soil and land vegetation is included in Figure 1 in the OP – about 60 Gt out of the soil directly, 60 Gt out of vegetation, and about 121.3 absorbed by both vegetation and soils. There’s also a net flux into forests of 0.5 Gt and a net flux into the atmosphere due to wildfires of 1.6 Gt. (why NASA separated the two separate vegetation absorption fluxes isn’t clear to me, but they did). Add up all the absorption and emission fluxes and you get 121.8 Gt flux into vegetation and soils and 121.6 Gg flux out of vegetation and soils, for a net absorption of 0.2 Gt.
Scientists have measured the flux in plots of land and then extrapolated that flux globally (with the appropriate uncertainty applied) and calculated that carbon dioxide absorption by soils directly and by land plants exceeds emissions. That’s represented on the graph.
And you’re right that the oceans store WAY more carbon than either the atmosphere or land soils (geologic features store WAY more than the oceans, but it’s not accessible to the carbon cycle on the time scales we’re talking about here). And oceanic outgassing has been the cause of prior climate variations. But there are two ways we know that’s not the source this time – the fact that dissolved carbon dioxide is increasing and the drop in atmospheric oxygen. If the oceans were the source of the carbon dioxide, then dissolved CO2 would be decreasing and atmospheric oxygen would be stable or increasing.
My logic for “the increase is all from fossil fuels” is admittedly a tad simplistic, but it comes from the fluxes shown in Figure 1. If you add up all the fluxes, you find that 0.2 Gt of carbon are being absorbed by terrestrial plants and soils, 2 Gt are being absorbed by the ocean, and 5.5 Gt are being emitted by humans burning fossil fuels. That means the net fossil fuel flux into the atmosphere is 3.3 Gt. Thus my “all of it” statement.
If you want to argue that it’s not quite that simple, then of course I agree. But the statement is accurate to the first order. Separating changes in the nearly balanced fluxes into various sinks would be a second or third order effect, and something that’s beyond the basic educational level at which I’m trying to keep the entire “Climate Science for Everyone” series.
About half of what is burned into the air is taken up by carbon sinks. There are serious questions that need answering in order to nail down just how much of the 100 ppm increase belongs to man. One person stated “nearly all of it”. I find that very hard to swallow. The soil carbon sink needs to be thoroughly analyzed to vouch for the isotopic ratios, especially with respect to soil bacterial/decay methane production. I may need to research more here. Generally, a climate warming period with a slow natural upward increase in atmospheric CO2 appears to be the norm The ocean’s ability to out gass CO2, one would expect then, should far exceed anything produced by man. If true that nearly all of it does, it would mean there has been a global temp downturn for the last century with man made CO2 warming offsetting this “unnoticed” cooling. Going further, spikes in global warming should then become more pronounced in the future. Is that happening? If global cooling is hidden, as suggested, but continues, it will overtake all the warming trends and we will soon see a downturn in Global temps. The crucial deal now is will SC25 disappear and usher in a sharp drop in global temps. I support for more cleaning up the air, but not necessarily for climate reasons. The main concerns may shift to the health of the human brain. Kids, as young as 6 years old, are developing brain plaques and tangles similar to Alzheimer Disease by breathing Mexico City’s noxious air. Dogs too. This is not happening in the rural areas.
CptWayne, given that accumulated human emissions are approximately double the actual rise in atmospheric CO2, as you yourself state, why should it be hard to understand that pretty much all of the atmospheric rise is attributable to human emissions from fossil fuel burning? The simple mass balance argument makes a pretty persuasive case; the isotopic ratios nail it. The change in 13C concentration is far greater than anything ever seen during “natural” 100ppm changes in atmospheric concentrations.
If you want to believe that something other than fossil fuel burning is responsible for a large part of the rise, your theory would need to explain
– what the extra source is
– why it started at the same time as humans started burning fossil fuels
– why it has a 13C content lower than the previously existing atmospheric CO2 but similar to CO2 from fossil fuel burning
– where the fossil fuel CO2 is going that you believe is not going into the atmosphere
Haaa, Haaa, Haaa,
That’s good!
[Snip]
[Admin: LOL in Oregon, the S&R comment policy says “We moderate our comments and commenters exhibiting bad faith behavior will have their offending comments deleted and they may be banned, possibly without warning. When evaluating the merit of a questionable comment, we will also consider whether said comment seeks to make any meaningful engagement the substance of the original post and/or subsequent comments in the thread.”
This comment has been deleted due to multiple violations of these expectations.]
Excellent!
It is OK to malign people who don’t agree with you, but when they respond:
“off with their heads!”
Have fun, Brian, espousing that the 5.5gt (by the way, it appears that the IPCC says it is 29GT)
amongst the 750 gt.
….and good luck with your eco-religion!
But then, this is so funny I have grist for some of the newspaper sites.
Remember, never say anything you wouldn’t want to see on the NY Times, LA Times, etc!
If you had argued in good faith, Lol, you wouldn’t have had your last comment deleted by the admin.
I’ve said nothing to be ashamed of, Lol. I’ve supported my arguments and merely demanded that you do the same. You have refused to do so and you committed at least two clear fallacies in the process – shifting the goalposts (Svensmark) and appealing to a misleading authority (your anonymous friend). And when you were called on it, you simply ignored your critics.
So if you want to put my words in front of newspapers, go for it. I’ve commented at some of them from time to time, and some of my posts have been picked up via syndication and published on those very sites. I have no fear of them.
So according to some, there is no increase in CO2 from manmade sources. I have two questions:
1. All the CO2 from the gigatons of carbon that we have been burning goes where?
2. The increase in CO2 concentration in the atmosphere comes from where?
For a hint to the answer to number 1, see question number 2. For a hint to the answer to question number 2, see question number 1. Here is another hint on the second question: The CO2 concentration in the ocean is increasing, making it more acidic, which is threatening coral and other ecosystems.